CONSORTIUM
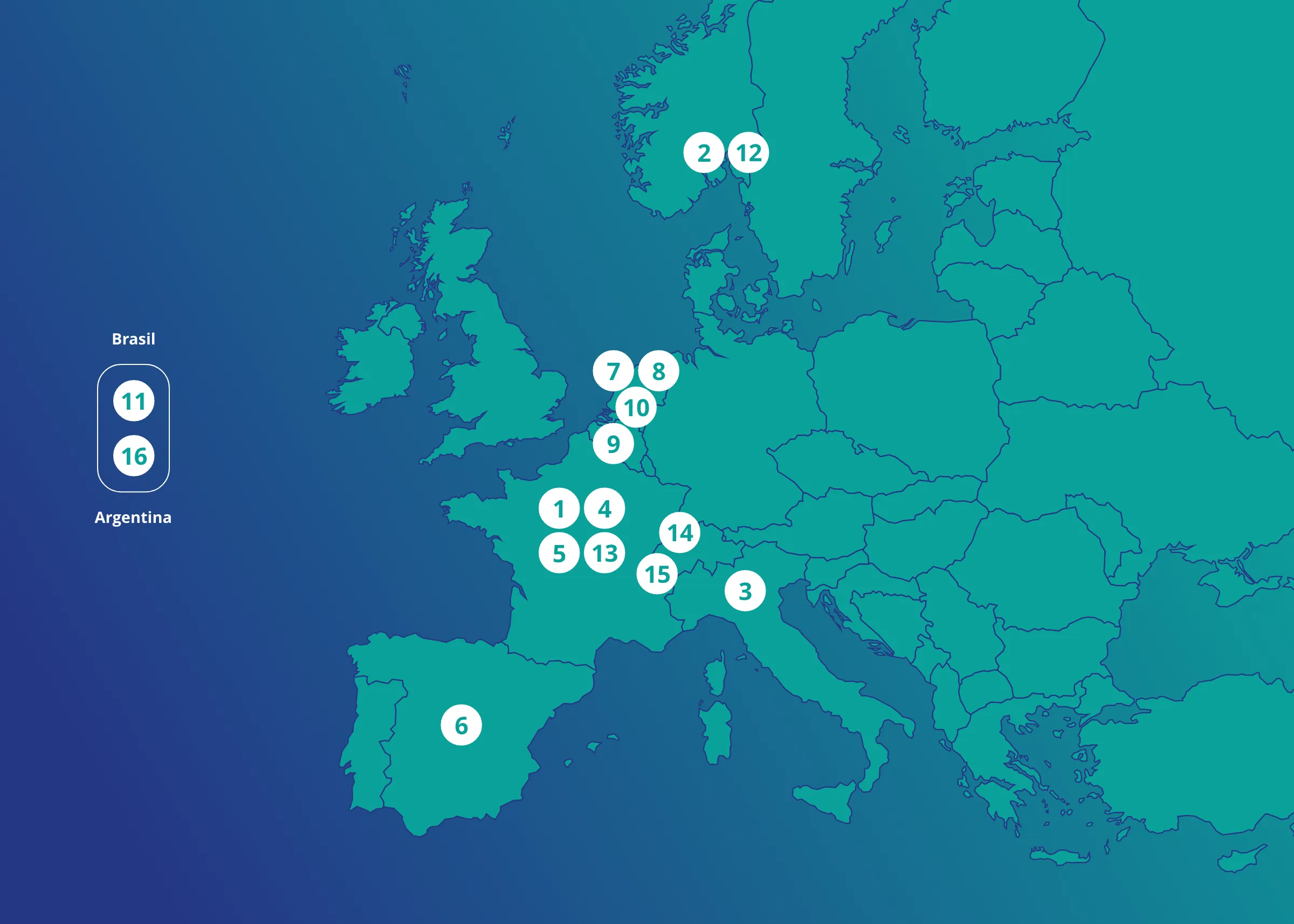
INSERM – ANRS MIE
Paris, France
Oslo Universitetssykehus HF
Oslo, Norway
Universita degli studi di Verona
Verona, Italy
Inserm Transfert SA
France
Assistance Publique des Hôpitaux de Paris
Paris, France
Servicio Madrileno de Salud
Madrid, Spain
European Clinical Research Alliance on Infectious Diseases
Utrecht, Netherlands
UNIVERSITAIR MEDISCH CENTRUM UTRECHT
Utrecht, Netherlands
UNIVERSITEIT ANTWERPEN
Antwerpen, Belgium
ERASMUS UNIVERSITAIR MEDISCH CENTRUM ROTTERDAM
Rotterdam, Netherlands
FUNDACAO OSWALDO CRUZ
Brazil
Folkehelseinstituttet
Norway
PANTHER
France
UNIVERSITAT BASEL
Basel, Switzerland
University of Geneva
Geneva, Switzerland
Fundación Huésped
Argentina














































